Explore innovative options for disc herniation surgery at our Englewood location.
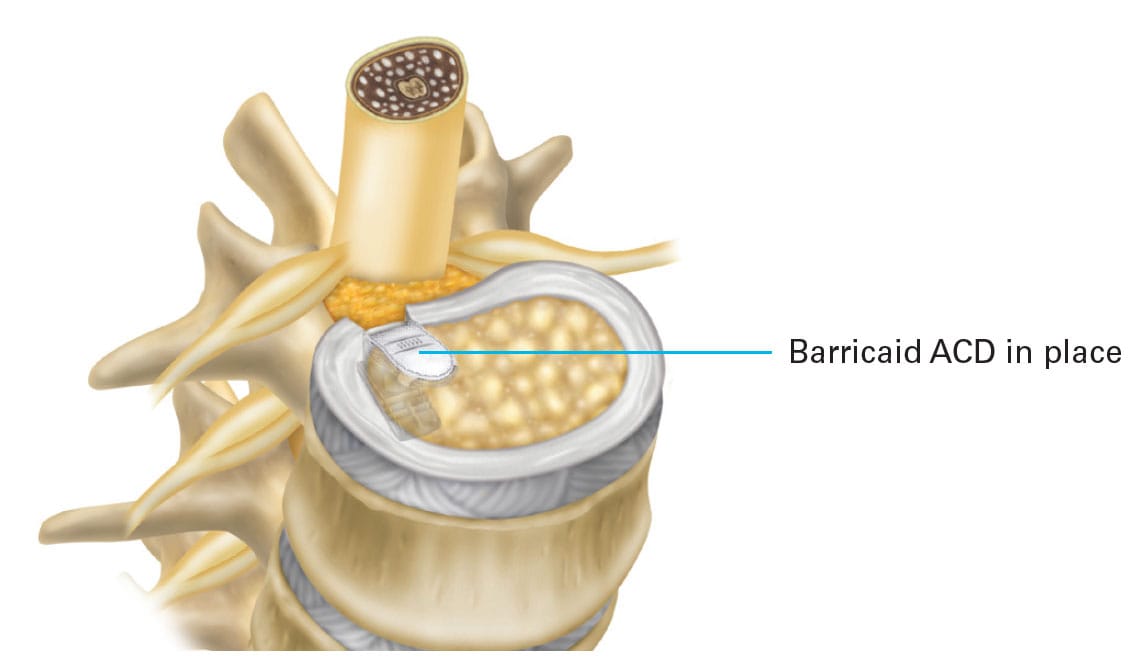
How the Barricaid Device Works
Disc herniations occur when the nucleus (the softer center) pushes through a weak spot or tear in the annulus (the tougher outer layer). While small slit-like defects pose a relatively low risk for future herniations, a larger defect can raise that risk to around 25%. Barricaid addresses this vulnerability by sealing the defect and anchoring to the vertebra, creating a protective barrier that reduces the chance of additional herniation.
Contact us today to learn more about whether adding Barricaid to your microdiscectomy may enhance your surgical outcome.